US Quiz of the Month – setembro 2025
Case Report
A 72-year-old man with a history of hypertension, dyslipidemia, benign prostatic hyperplasia, gout, and a prior ST-elevation myocardial infarction (on clopidogrel) was referred for further evaluation following an abnormal computed tomography (CT) finding.
Clinically, the patient reported changes in bowel habits, with altered consistency, along with recent constitutional symptoms including <10% weight loss and anorexia. He denied current fever, abdominal pain, gastrointestinal bleeding, or other systemic symptoms.
Routine laboratory work-up was unremarkable, with normal thyroid and negative viral serologies.
CT imaging demonstrated focal thickening of the gastric wall along the greater curvature, with no associated lymphadenopathy or intra-abdominal abnormalities.
Given these findings, esophagogastroduodenoscopy was performed, revealing a 10-mm subepithelial protrusion along the greater curvature of the stomach, covered by intact mucosa (Figure 1). Mucosal biopsies (bite-on-bite technique) were non-diagnostic.
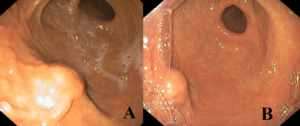
Figure 1. Upper endoscopy findings. (A, B) Subepithelial bulging along the greater curvature of the stomach, covered by normal mucosa.
Endoscopic ultrasound (EUS) (Figure 2) demonstrated a well-defined, heterogeneous, hypoechoic lesion, measuring approximately 15 × 15 mm, arising from the fourth layer (muscularis propria), without cystic change, calcification, or regional lymphadenopathy. Color Doppler imaging revealed no significant vascular flow.
The first fine-needle biopsy (FNB) was non-diagnostic, and a repeat EUS-guided biopsy was subsequently performed. FNB was carried out using a 22G Acquire and SharkCore needle (Covidien) via the transgastric route, with two passes each, employing the slow-pull technique. The lesion was firm on needle puncture.
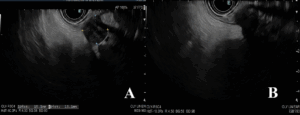
Figure 2. Endoscopic ultrasound findings. A well-demarcated, heterogeneous, hypoechoic lesion with small anechoic areas, measuring approximately 15 × 15 mm.
Histological examination (Figure 3) revealed a spindle-cell proliferation with peripheral lymphoid cuffing, while immunohistochemistry demonstrated sAddonstrong S-100 positivity and negativity for actin, desmin, cytokeratins, chromogranin, CD117, and DOG1.
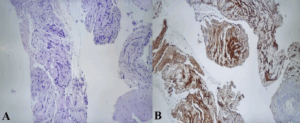
Figure 3. Histopathological and immunohistochemical findings. Spindle-cell proliferation with peripheral lymphoid cuffing (H&E) – A, and diffuse positivity for S-100 – B.
What is the most likely diagnosis?
Discussion
Gastric schwannomas (GS) are rare, benign, slow-growing mesenchymal tumours arising from Schwann cells of the nerve sheath within the gastrointestinal wall, most commonly from Auerbach’s or Meissner’s plexus. They account for approximately 3% of all gastrointestinal mesenchymal tumours and only 0.2% of all gastric neoplasms. These tumours usually present as solitary lesions located in the submucosa or muscularis propria, most frequently in the body of the stomach (50%), followed by the antrum (32%) and fundus (18%).
GS are typically benign, with an excellent prognosis and extremely low malignant potential. Because their clinical presentation is nonspecific, they are most often discovered incidentally during radiological or endoscopic investigations performed for unrelated conditions.
Differentiation between GS and other gastric subepithelial tumours—particularly gastrointestinal stromal tumours and leiomyomas —can be challenging. EUS plays a key role in characterization, typically revealing a well-circumscribed, hypoechoic mass arising from the fourth layer (muscularis propria), with heterogeneous echogenicity and occasional internal high-echo areas. Cystic degeneration or calcification is uncommon, and when vascularity is present, it is generally mild and slow-flowing.
Histopathological and immunohistochemical evaluation is essential for establishing the diagnosis. GS characteristically exhibit a spindle-cell proliferation with peripheral lymphoid cuffing and an immunophenotype consistent with Schwann-cell differentiation. Immunohistochemistry typically shows strong nuclear and cytoplasmic positivity for S-100 protein, vimentin, and glial fibrillary acidic protein, with consistent negativity for CD117, DOG1, CD34, and smooth muscle actin, confirming their neural origin.
Management usually involves complete endoscopic or surgical resection, which is curative. Lymphadenectomy or adjuvant therapy is unnecessary, as GS are benign tumours with excellent long-term outcomes and extremely low recurrence rates.
References:
- Hong SW, Cho WY, Kim JO, Chun CG, Shim KY, Bok GH, et al. Gastric schwannoma diagnosed by endoscopic ultrasonography-guided Trucut biopsy. Clin Endosc. 2013;46(3):297–301.
- Zhong DD, Wang CH, Xu JH, Chen MY, Cai JT. Endoscopic ultrasound features of gastric schwannomas with radiological correlation: A case series report. World J Gastroenterol. 2013;19(3):495–501.
- Erdoğan D, Yüksel O, Gürler M, et al. Gastrointestinal schwannomas: A case series of 9 patients and literature review. Turk J Surg. 2025;41(2):85–92.
- Okai T, Minamoto T, Ohtsubo K, et al. Gastric schwannoma: Endoscopic ultrasonographic and computed tomographic findings. Gastrointest Endosc. 2003;57(3):395–398.
- Miettinen M, Shekitka KM, Sobin LH. Schwannomas in the colon and rectum: A clinicopathologic and immunohistochemical study of 20 cases. Am J Surg Pathol. 2001;25(7):846–855.
- Voltaggio L, Murray R, Lasota J, Miettinen M. Gastrointestinal schwannomas: Clinicopathologic study of 51 cases and critical review of the literature. Hum Pathol. 2012;43(5):650–659.
Authors
Margarida Portugal¹; Isabel Carvalho¹; Francisco Velasco¹; Marta Eusébio¹; Bruno Peixe¹
¹ Unidade Local de Saúde do Algarve – Hospital de Faro, Gastroenterologia


