US Quiz of the Month – December 2020
CASE REPORT
A 39-year-old woman presented with a two months history of epigastric pain partially relieved with proton pump inhibitors (PPI). She had no history of nausea, vomiting, diarrhoea or weight loss. She had no significant medical or family history. Physical examination was unremarkable. The workup revealed haemoglobin of 13.8 g/dL, albumin of 3.6 g/dL and fasting serum gastrin of 37.7 ng/mL. Serologic test for Helicobacter pylori (H. pylori) was positive. An upper gastrointestinal endoscopy revealed giant folds with a polypoid appearance in the greater curvature of the stomach body with sparing of the antrum (Fig. 1).

Figure 1. Endoscopic view with enlarged gastric folds, thick mucus and surface erosions on the greater curvature of the stomach.
Tissue specimens of altered gastric mucosa were obtained using standard biopsy forceps. Endoscopic ultrasound showed thickening of the mucosal layer of the stomach body with preservation of wall stratification. Antrum wall had normal thickness and structure (Fig. 2 and Video 1).
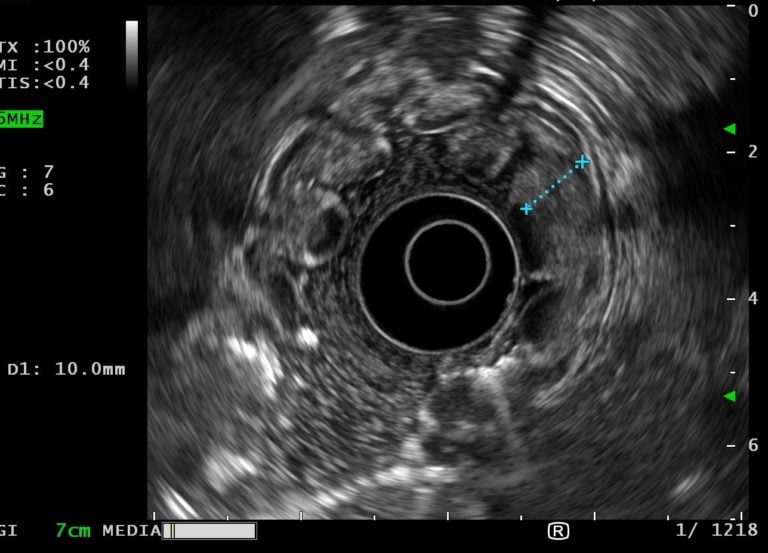
Figure 2. EUS showing a gastric wall with 10 mm with an echogenic thickening of the mucosal layer wall with preservation of normal architecture.
WHAT IS THE MOST LIKELY DIAGNOSIS?
DISCUSSION
Histology revealed foveolar hyperplasia, glandular tortuosity and dilatation, mucosal oedema and decreased number of parietal cells (Fig. 3). These features are consistent with the diagnosis of Ménétrier’s Disease (MD).
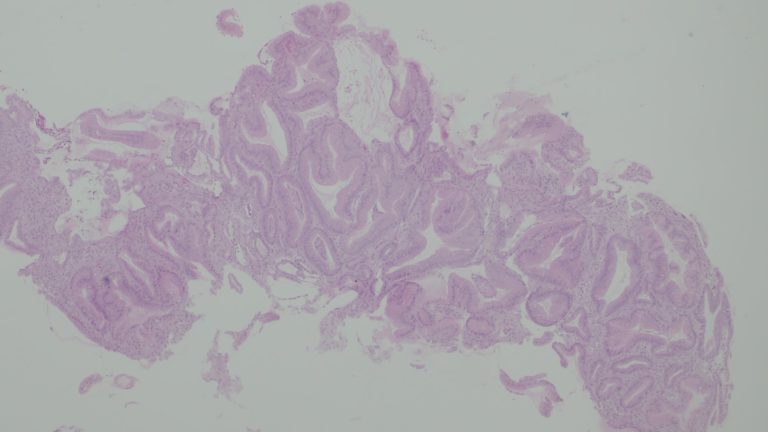
Figure 3. Histology revealing relative preservation of mucosal architecture with marked foveolar hyperplasia, tortuosity and dilatation oh the glands, smooth muscle and decreased number of parietal cells.
The patient was treated with concomitant therapy (PPI, amoxicillin, clarithromycin and metronidazole) for H. pylori eradication. She remains asymptomatic two months after the treatment and H. pylori eradication was verified with urea breath test. Additional invasive procedures were deferred because she became pregnant.
MD is a rare, idiopathic hypertrophic gastropathy characterized by giant rugal folds in the gastric body, foveolar hyperplasia and markedly decreased or absent oxyntic glands with antral sparing [1]. The pathogenesis is related to increased EGFR signalling in the stomach. Citomegalovirus (CMV) and H. pylori infections have been associated with MD. Classical presentation includes abdominal pain, nausea, and vomiting and peripheral oedema due to protein loss across the gastric mucosa with resultant low albumin. Upper gastrointestinal endoscopy shows enlarged folds usually confined to the oxyntic mucosa with sparing of the antrum [1,2]. A full-thickness gastric biopsy is usually required to document the loss of the deep glandular component. EUS is a useful procedure in the differential diagnosis with other causes of enlarged gastric folds. A massive thickening of the mucosal and/or submucosal layer, with increased echogenicity seems to be a characteristic EUS feature [3,4]. MD should be distinguished from other diseases characterized by enlarged gastric folds, such as, gastric polyposis syndrome, hyperplasic gastritis, Zollinger-Ellison Syndrome and gastric malignancy [1]. Multiple medical treatments including PPI, H. pylori/CMV treatment, cetuximab and octreotide have been used in patients with MD, however, controlled trials are lacking and most pharmacologic treatments do not seem to provide consistent benefits. Gastrectomy is reserved for those patients refractory to medical therapy. The increased risk of gastric neoplasia in these patients is uncertain, however, some authors recommend annual endoscopic surveillance [5].
REFERENCES
- Rich A, Toro T, Tanksaly J, Fiske W, Lind C, Ayers G, et al.: Distinguishing Ménétrier’s Disease from its mimics. Gut 2010;59:1617-1624.
- Yang L, Zhao X, Li A, Sheng J: Ménétrier’s disease with normal albumin level. Endoscopy 2015:9-10.
- Hizawa K, Kawasaki M, Yao T, Aoyagi K, Kawakubo K, Fujishima M: Protein-losing gastropathy with hypertrophic gastric folds. Endoscopy 2000;32:394-397.
- Gur M, Besir F, Ermis F: Computed tomography view of Ménétrier’s Disease. J Gastrointestin Liver Dis. 2014;23(4):364.
- Toubia N, Schubert M: Ménétrier’s Disease. Curr Treat Options Gastroenterol 2008;11:103-108.
AUTHORS
Mafalda João1, Luís Elvas1, Susana Alves1, Ana Teresa Cadime1
- Gastroenterology Department, Portuguese Oncology Institute of Coimbra, Portugal


