US Quiz of the Month – November 2019
CASE REPORT
A 55-year-old female, smoker, with a history of hypertension was admitted to the emergency department with an oedematous acute pancreatitis. She improved after conservative treatment but maintained symptoms of epigastric pain aggravated by food ingestion and vomiting. An upper endoscopy revealed gastric erythema and biopsies were compatible with a chronic gastritis. On abdominal ultrasound no hepato-biliary-pancreatic lesions were found. Despite medication with proton pump inhibitor, pancreatine and alcohol/tobacco abstinence, she kept complaints of epigastalgia, nausea and a loss of 5 Kg. Additional workup with magnetic resonance imaging (MRI) showed a nodular wall thickening of the second portion of the duodenum, having contrast enhancement, with 15x17x14mm (LxTxPA) – Fig 1. CEA and Ca 19.9 were normal.
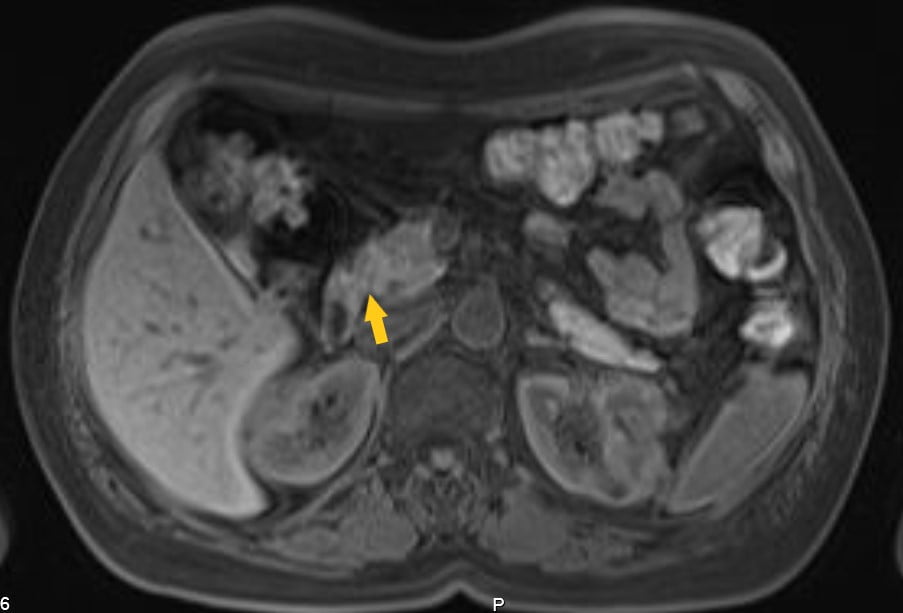
Figure 1. MRI showing nodular wall thickening of D2
An endoscopic ultrasound (EUS) was performed and showed areas of duodenal wall thickening in D2 and D3, with infracentimetric hypoechoic foci. The pancreatic parenchyma was heterogenous in the pancreatic head and uncinate process, with hyperechoic striae (honeycomb pattern) and hypoechoic lobulations; pancreatic duct was normal.
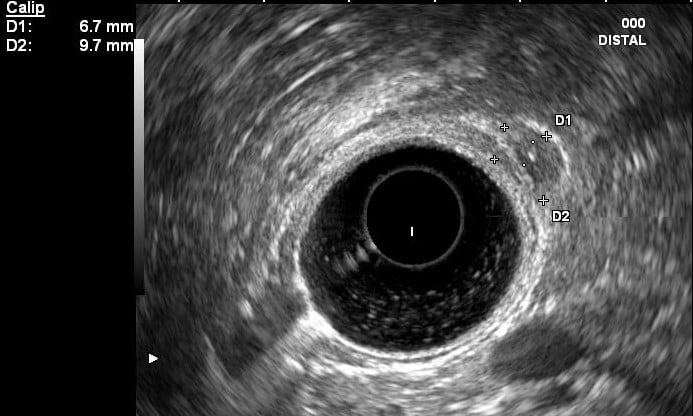
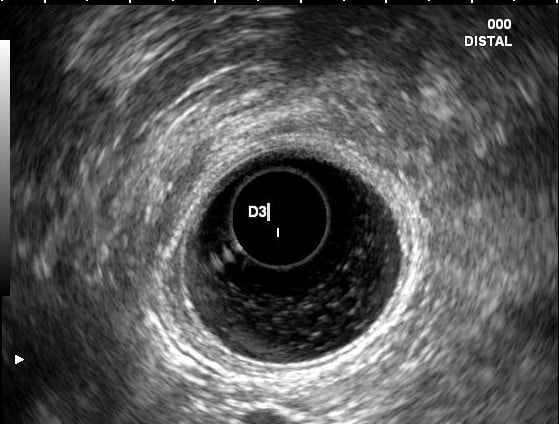
Figures 2 and 3. EUS showing hypoechoic thickening of the D2 and D3 wall and a hypoechoic foci
WHAT IS THE MOST LIKELY DIAGNOSIS?
DISCUSSION
A diagnosis of paraduodenal pancreatitis (PP) was assumed and the patient was started on octreotide.
Paraduodenal pancreatitis has been defined as a distinct subset of chronic pancreatitis involving the duodenal wall in the region of the minor papilla. (1,2) Patients are predominantly males, with 40-50 years old and a history of alcohol abuse and heavy smoking. (1,2,3)
The pathogenesis of PP is yet to be clarified although the available evidence highlights the presence of pancreatic tissue within the duodenal wall, which may result from abnormal migration of the dorsal pancreatic bud, and the effect of alcohol intake and cigarette smoking on this heterotopic pancreas. (3)
The macroscopic pathologic features include thickening of the duodenal wall near the minor papilla and eventually cystic components. (1) These findings are usually seen in the submucosa and muscularis mucosae, may extend to the“groove” zone delimited by the duodenum, pancreas, and common bile duct. The inflammatory process can also involve the adjacent pancreatic tissue and the main pancreatic duct, with upstream dilation leading to an obstructive chronic pancreatitis, defined as “diffuse form” or be limited to the groove zone, without proper pancreatic involvement – “pure form”. The latter is reported to occur in 18.3 to 33%. (1,2)
Upper abdominal pain, often continuous and severe, is the most frequent and relevant symptom in PP. Vomiting and jaundice may occur due to involvement of the duodenum or the common bile duct. Weight loss is the consequence of these symptoms, sometimes associated with steatorrhea and diabetes. These symptoms are shared with those of periampullary cancers. (1,2) Pancreatic carcinoma arising in the groove region is one of the most particularly challenging differencial diagnosis; others include cystic tumors, duodenal carcinoma, cholangiocarcinoma, autoimmune pancreatitis and acute relapsing pancreatitis. Tumour markers, such as carcinoembryonic antigen (CEA) and carbohydrate antigen (CA) 19-9, are usually normal in PP. (2)
MRI and CT scan have a good correlation with pathological findings, showing fibrosis of the groove space and duodenal thickening with/without cysts. EUS is considered the preferred method as it may reveal a hypoechoic area between the duodenal wall and the pancreas, thickening and stenosis of the duodenum, intramural cysts and signs of chronic pancreatitis. EUS-FNA has limited value for the wide range of possible results, which may be misinterpreted as a tumor, especially if there is an abundance of Brunner gland hyperplasia and spindle cells. (2,3)
The initial therapeutic approach comprises alcohol/tobacco withdrawal, diet and analgesics (1,3). Somatostatin analogs and endoscopic approach (stricture dilation and cysts or pancreatic ductal drainage) have been reported to relief symptoms. (2,3) Pancreaticoduodenectomy improves clinical outcomes and remains the first treatment option in patients with a duodenal obstruction or when a diagnosis of cancer cannot be ruled out. (1)
REFERENCES
- Pretis N, Capuano F, Amodio A, Pellicciari M, et al; Clinical and Morphological Features of Paraduodenal Pancreatitis: An Italian Experience With 120 Patients; Pancreas 2017;46: 489–495.
- Arora A, Dev A, Mukund A, Patidar Y, et al; Paraduodenal pancreatitis; Clinical Radiology 2014; 69: 299-306.
- Carvalho D, Loureiro R, Pavão V, Russo P et al; Paraduodenal Pancreatitis: Three Cases with Different Therapeutic Approaches; GE Port J Gastroenterol 2017;24:89–94.
AUTHORS
Sara Santos1, Diana Carvalho1, Gonçalo Ramos1
- Gastroenterology Department, Centro Hospitalar Universitário de Lisboa Central, Lisbon, Portugal


