US Quiz of the Month – September 2020
CASE REPORT
A 49-year-old, caucasian, healthy man underwent an abdominal ultrasound due to nonspecific abdominal complaints, which revealed an incidental finding in the uncinate process of the pancreas of a nodular area with 26×24 mm. Physical examination was normal. Laboratorial data was unchanged, namely CA 19-9. In addition, he performed other complementary diagnostic tests. Abdominal computed tomography (CT) showed a hypodense, hypovascular focal lesion in the uncinate region of the pancreas, 28 mm in diameter, without Wirsung dilation. Magnetic resonance imaging (MRI) with cholangiography revealed a cystic image with heterogeneous content in the uncinate process, measuring 30 mm, with thickened walls, contacting the terminal portion of the pancreatic duct without dilatation (Fig. 1).
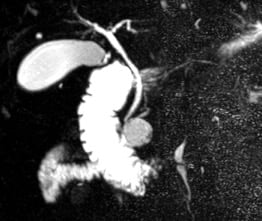
Figure 1. MRI cholangiography: cystic image with heterogeneous content in the uncinate process, measuring 30 mm, with thickened walls, contacting the terminal portion of the pancreatic duct without dilatation.
Endoscopic ultrasonography (EUS) identified in the uncinate process, a cystic lesion of 26 mm, with solid content, marked wall thickening and increased peripheral vascularization. No communication with the Wirsung duct, nor vascular invasion was detected. Fine needle aspiration (FNA) of the cyst and biopsy of the wall with micro forceps through the 19G needle were also performed (Fig. 2 and 3) (Video 1).
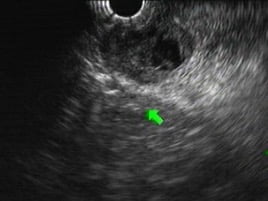
Figure 2. EUS: cystic lesion located in the uncinate process, with solid content, marked wall thickening.
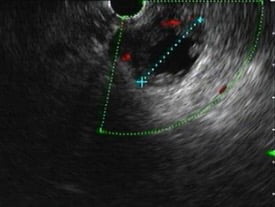
Figure 3. EUS: cystic lesion located in the uncinate process, measuring 26 mm and with increased peripheral vascularization.
WHAT IS THE MOST LIKELY DIAGNOSIS?
DISCUSSION
The cytohistological description revealed monomorphic cells showing with little cohesion and with scarce, clarified or eosinophilic cytoplasm, sometimes foamy, and round to oval nuclei with indistinct nucleoli. The cells were organized in solid aggregates and in a slightly radial arrangement around fibrovascular axis (Fig. 4).

Figure 4. Pathology (H&E, X400): monomorphic cells showing with little cohesion and with scarce, clarified or eosinophilic cytoplasm, sometimes foamy, and round to oval nuclei with indistinct nucleoli. The cells were organized in solid aggregates and in a slightly radial arrangement around fibrovascular axis.
The results of the immunohistochemical study were positive for vimentin (diffuse), beta-catenin (nuclear and cytoplasmic) and negative for chromogranin and estrogen receptors (Fig. 5). The pathology final report made the diagnosis of a solid pseudopapillary tumor (SPT) of the pancreas.
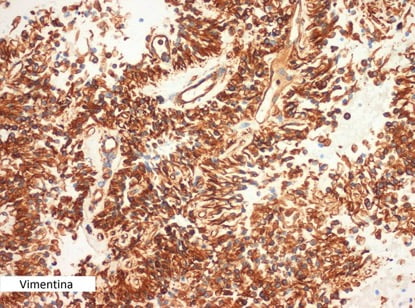
Figure 5. Pathology (Vimentin, X400): immunohistochemical study was positive for vimentin.
SPT of the pancreas was first described by Frantz in 1959. It is a rare neoplasm and accounts for 5% of the resected pancreatic cystic tumors. It usually occurs in young women, with an incidence peak between the second and third decades of life. The clinical presentation is non-specific, with abdominal pain being the main form of presentation in 67% to 81% of cases. The diagnosis is made through imaging methods, namely abdominal ultrasound, CT, MRI and EUS. 1,2
EUS is a useful complementary method for diagnosis and when associated with FNA guarantees a more accurate diagnosis. SPTs can be located in the head, body or tail of the pancreas, occurring preferably in the body and tail, usually are cystic lesions, unique, well delimited, with central hemorrhagic cystic degeneration with solid and cystic appearance.2
The immunohistochemistry neoplastic cells usually present positive markers for vimentin, α-1 antitrypsin and α-1-antichymotrypsin, β-catenin, CD10 and CD56, and sometimes express focal positivity for specific neuron enolase, synaptophysin and progesterone receptors.3 They present low potential for malignancy, but local and remote invasion may occur; the liver being the most common site of metastasis.1
Due to the benign nature of the SPTs and the excellent postoperative prognosis, surgical resection is considered the treatment of choice for these lesions.1 Our patient was submitted to a Whipple resection and the diagnosis of SPT was confirmed in the surgical specimen. After 6 months of follow-up, the patient was asymptomatic and without evidence of neoplastic disease.
REFERENCES
- Feldman, Mark, Friedman, Lawrence S., Brandt, Lawrence J., Sleisenger and Fordtran’s Gastrointestinal and liver disease 2010; 60: 1042-1043
- Hawes, Robert H., Fockens, Paul, Varadarajulu, Shyam, Endosonography 2015; 16: 220-221
- Gress, Frank G., Savides, Thomas J., Endoscopic ultrasonography 2016; 19: 174, 176
AUTHORS
Giza Mirian1; Pedro Bastos1
- Gastroenterology Department, Instituto Português de Oncologia – Porto, Porto, Portugal.


