US Quiz of the Month – abril 2025
Case Report
A 36-year-old male, mechanic, with no known past medical history presents with diffuse abdominal pain, anorexia, asthenia, and a dry cough. He denied vomiting, jaundice, dyspnea, fever, night sweats, weight loss, and altered bowel habits. He also denied any joint, dermatologic or ophthalmologic complaints or sicca syndrome.
A chest CT scan showed multiple hilar and mediastinal adenopathies and micronodular infiltrates in the upper pulmonary lobes, as well as multiple subpleural nodularities, most prominent in the upper half of the lung fields.
He was evaluated by the pulmonology department with unremarkable pulmonary function tests and inconclusive analytical studies, including a negative erythrocyte sedimentation rate, angiotensin-converting enzyme at the upper limit of normal, and negative ANA, ANCA, reumathoid factor and anti-CCP. He was also negative for avian, pigeon, and Aspergillus precipitins, IGRA, and HIV.
He underwent endobronchial ultrasound (EBUS) without macroscopic changes and a puncture was performed in station 7. Histological results showed no obvious granuloma formation and no specific changes. He also had a bronchoalveolar lavage and aspirate without epithelioid macrophages or multinucleated giant cells. Microbiology was negative.
Given the inconclusive results, he underwent a repeat chest CT, which was similar to the previous one, and a 18F-FDG PET scan, which showed a 51.2mm adenopathy conglomerate in the celiac-mesenteric region (SUV max 12.4).
Therefore, the patient underwent an endoscopic ultrasound (EUS) that revealed a conglomerate of hypoechogenic adenopathies with an elongated morphology, adjacent to the emergence of the celiac trunk, the largest with a long axis of 27mm, posterior to the splenic vessels, and another of 17mm anterior to the splenic artery (Figure 1A-C), where a fine needle biopsy (FNB) was performed (Figure 1D), using a 22G needle (Medi-Globeâ). Another smaller adenopathy (9mm) was found nearby (Figure 1B). There were no other pathological findings.
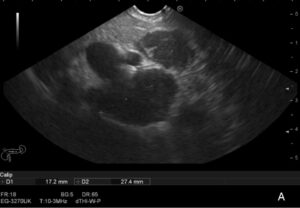
Figure 1 A

Figure 1 B

Figure 1 C
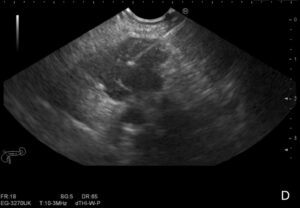
Figure 1 D
Figure 1. Adenopathy conglomerate in the celiac-mesenteric region identified on EUS (1A-C), where a EUS-FNB was performed (1D).
What is the most likely diagnosis?
Histological analysis showed multiple epithelioid granulomas without necrosis and the presence of multinucleated giant cells with peripherally arranged nuclei, highly suggestive of sarcoidosis.
Discussion
Sarcoidosis is a multisystem granulomatous disease of unknown cause, mainly affecting the lungs and lymph nodes, characterized by noncaseating granulomatous lesions and generally occurs between the ages of 20 and 40. [1,2]
Patients may present with a variety of symptoms, including constitutional symptoms, respiratory symptoms, adenopathy (LNs), granulomatous uveitis, erythema nodosum and hypercalcemia. [1,2]
The diagnosis of sarcoidosis remains a complex and often lengthy process due to the need for invasive procedures and the lack of a definitive diagnostic test. It typically requires a combination of clinical, radiologic and histopathologic evaluation and the exclusion of alternative causes of granulomatous disease, such as malignancy and infectious diseases, including lymphoma, tuberculosis and Wegener’s granulomatosis. [1,2]
Since the lungs and mediastinal LNs are the most commonly involved sites, EBUS-guided transbronchial biopsy (EBUS-TBB) is recommended in most cases and has a diagnostic yield of 40% to 90% when 4 to 5 biopsies are performed. [1,3]
If transbronchial biopsies are nondiagnostic, other accessible biopsy sites, if available, should be evaluated. The differential diagnosis in occasional cases where sarcoidosis presents as intra-abdominal LNs or masses, is extensive, and EUS-guided fine needle aspiration (EUS-FNA) is a potentially useful modality to establish the definitive diagnosis and direct treatment, with a diagnostic yield of 86%. [3]
Knowing the advantages of EUS-fine needle biopsy (EUS-FNB), compared to EUS-FNA, it is expected that EUS-FNB will provide high diagnostic yield in patients with sarcoidosis. The safety profile is acceptable, although there is a slight risk of infectious complications. EUS-FNA/FNB, a minimally invasive and well tolerated procedure, offers a viable alternative to EBUS-TBB for the diagnosis of sarcoidosis. [4]
The authors present this case to illustrate the usefulness of EUS-FNB in the diagnostic process of sarcoidosis.
References
1.Statement on Sarcoidosis. American Journal of Respiratory and Critical Care Medicine. 1999;160(2):736-55.
2. Waly YM, Sharafeldin A-BK, Akhtar MU, Chilmeran Z, Fredericks S. A review of sarcoidosis etiology, diagnosis and treatment. Frontiers in Medicine. 2025 Feb 26;12. doi:10.3389/fmed.2025.1558049
3. Michael H, Ho S, Pollack B, Gupta M, Gress F. Diagnosis of intra-abdominal and mediastinal sarcoidosis with EUS-guided FNA. Gastrointest Endosc. 2008 Jan;67(1):28-34. doi: 10.1016/j.gie.2007.07.049
4. Shinohara Y, Oki M. Transesophageal endosonography in the diagnosis of sarcoidosis: A narrative review. Mediastinum. 2024 Dec;8:50–50. doi:10.21037/med-24-37
Authors
Isabel Caetano1, Plácido Gomes1, Diogo Simas1, Christine Costa2, Maria Fernanda Cunha3, Alexandra Fernandes1, Helena Vasconcelos1
1 – Department of Gastroenterology, Unidade Local de Saúde da Região de Leiria, Leiria, Portugal.
2 – Department of Pulmonology, Unidade Local de Saúde da Região de Leiria, Leiria, Portugal.
3 – Pathology Department, Unidade Local de Saúde da Região de Leiria, Leiria, Portugal.


