US Quiz of the Month – agosto 2025
Case Report
A 74-year-old male with previous history of diverticulosis presented to the emergency department for the third time in a month with complaints of hematochezia. Patient reported a 10kg weight loss in the past month. Blood test results revealed hemoglobin 11.7g/dL. Colonoscopy showed an elevated 30mm lesion with central mucosal disruption and active bleeding, located at the splenic flexure of the colon (Fig 1A-B). Endoscopic hemostasis was achieved with diluted adrenaline, through-the-scope clips, and hemostatic powder (Fig 1C-D). Biopsies were performed but inconclusive. CT angiography revealed a 7.5 x 5.5 cm lobulated, exophytic lesion with peripheral enhancement and central hypodense areas, extending from the transverse colon and in direct contact with the pancreatic tail (Fig2). This also showed perilesional adenopathies and multiple liver metastases.
An endoscopic ultrasound (EUS) was performed for further characterization and tissue sampling was done using a fine 22 G needle biopsy (FNB). The referred lesion appeared hypoechoic and heterogeneous with internal anechoic areas (Fig3).
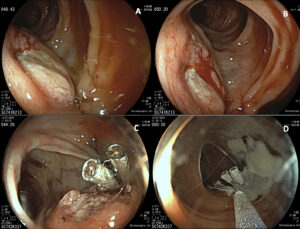
Figure 1 : Colonoscopy images. A and B: Elevated lesion with central mucosal disruption and active bleeding, located at the splenic flexure of the colon; C and D: Endoscopic hemostasis was achieved with diluted adrenaline, through-the-scope clips, and hemostatic powder.
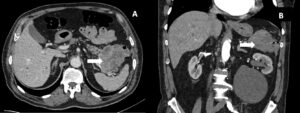
Figure 2: Transverse (A) and sagittal (B) CT images showing a lobulated, exophytic lesion (arrow) with peripheral enhancement and central hypodense areas, extending from the transverse colon and in direct contact with the pancreatic tail
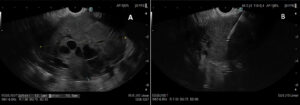
Figure 3: EUS shows a hypoechoic and heterogeneous mass with internal anechoic areas (A). Tissue sampling was performed using 22 G fine needle biopsy (FNB) (B)
What is the most likely diagnosis?
Discussion
Pancreatic ductal adenocarcinoma (PDAC) is the most common primary malignancy of the pancreas, accounting for over 90% of pancreatic cancers1. It frequently arises in the pancreatic head but may also develop in the body or tail, often presenting late due to the retroperitoneal location. Typical symptoms include weight loss, abdominal or back pain, and jaundice when the biliary tree is obstructed. Gastrointestinal bleeding is uncommon and usually indicates local invasion of adjacent hollow viscera, as in this case.
Cross-sectional imaging frequently demonstrates an ill-defined, hypodense mass with heterogeneous enhancement and features of vascular encasement or invasion of nearby structures2. Endoscopic ultrasound (EUS) is highly sensitive for detecting and characterizing pancreatic lesions and allows for fine-needle aspiration or biopsy, which remains the gold standard for diagnosis3. In this patient, EUS demonstrated a heterogeneous, hypoechoic lesion with central anechoic areas, and histopathology confirmed PDAC.
The main differentials for a large exophytic mass invading the colon include gastrointestinal stromal tumor (GIST), lymphoma, and pancreatic neuroendocrine tumor (NET). GISTs are typically hypervascular, well-defined, and arise from the muscularis propria of the GI tract, often positive for KIT (CD117) and DOG14. Lymphomas present as bulky, homogeneous masses with minimal desmoplastic reaction and usually lack necrosis5. Pancreatic NETs are often smaller, well-circumscribed, and hyperenhancing on imaging6. Pancreatic adenocarcinoma carries a poor prognosis, with overall 5-year survival rates below 10%1. Surgical resection is the only potentially curative option, but most patients present with locally advanced or metastatic disease. Management involves multidisciplinary care, including systemic chemotherapy and palliative interventions when resection is not feasible. This patient was proposed for palliative chemotherapy with Gemcitabine-Nabpaclitaxel.
References
- McGuigan A, Kelly P, Turkington RC, et al. Pancreatic cancer: A review of clinical diagnosis, epidemiology, treatment and outcomes. World J Gastroenterol. 2018;24(43):4846–4861.
- Elta GH, Enestvedt BK, Sauer BG, Lennon AM. ACG Clinical Guideline: Diagnosis and Management of Pancreatic Cysts. Am J Gastroenterol. 2021;116(5):1107–1128.
- de Moura DTH, McCarty TR, Jirapinyo P, et al. EUS-FNA versus EUS-FNB for pancreatic mass sampling: a systematic review and meta-analysis. Endosc Int Open. 2020;8(4):E281–E293.
- Søreide K, Sandvik OM, Søreide JA, et al. Gastrointestinal stromal tumors (GISTs): A review of risk assessment and prognostic factors. World J Gastrointest Surg. 2016;8(11):735–745.
- Bhatia A, Kumar Y, Kathuria S. Gastrointestinal lymphoma: A review. J Transl Intern Med. 2020;8(2):107–118.
- Shah MH, Goldner WS, Benson AB, et al. Neuroendocrine and Adrenal Tumors, Version 2.2021, NCCN Guidelines. J Natl Compr Canc Netw. 2021;19(7):839–868.
Authors
Pedro Miguel Rocha1, Filipa Lima1, Mafalda João1, Margarida Ferreira1, Pedro Narra Figueiredo1,2
- Gastroenterology Department, Hospitais da Universidade de Coimbra, Unidade Local de Saúde de Coimbra, Coimbra, Portugal
- Faculty of Medicine, University of Coimbra, Coimbra, Portugal


