US Quiz of the Month – novembro 2025
Case Report
The authors present the case of a 53-year-old woman with a medical history significant for lobular breast carcinoma (T3 N0 M0) diagnosed three years earlier, for which she underwent surgical treatment followed by adjuvant hormonal therapy with tamoxifen, which was ongoing at the time of presentation. Genetic testing performed at that time (BRCA1, BRCA2, CDH1, and TP53) was negative. She had no family history of cancer. The patient had been under surveillance, including tumor markers, breast ultrasound, and whole-body PET-CT (FDG), without any evidence of disease recurrence.
The patient presented with a two-month history of early satiety, heartburn, regurgitation, anorexia, and unintentional weight loss (3Kg). An upper GI endoscopy was performed, revealing an extensive, infiltrative, non-ulcerated gastric neoplasm involving the entire circumference of the fundus and body, with marked limitation of gastric distension – features typical of linitis plastica. Endoscopic biopsies were obtained and demonstrated a poorly cohesive carcinoma; however, the pathological material was scarse.
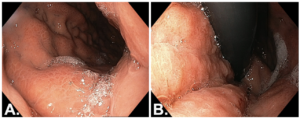
Figure 1. Upper GI endoscopy: A – Forward view of the gastric body. B – Retroflexed view of the gastric body.
Thoracic and abdominopelvic CT, as well as abdominal magnetic resonance imaging MRI, showed diffuse thickening of the gastric wall without evidence of distant metastatic disease. Whole-body PET-CT (FDG) did not reveal any abnormal radiotracer uptake.
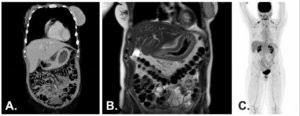
Figure 2. A – Thoracic and abdominopelvic CT (coronal view). B – Abdominal MRI (T2, coronal view). C – Whole-body PET-CT (FDG).
For further staging, EUS was performed. This revealed a hypoechoic thickening of the gastric wall measuring up to 10 mm, extending from the cardia to the antrum, sparing only the fundus and pylorus. There was loss of the normal layered structure of the gastric wall, with invasion beyond the muscularis mucosa. Five suspicious perigastric lymph nodes were identified; these were hypoechoic, round, despite being subcentimetric. Additionally, perigastric ascites was noted. The EUS staging was T3 versus T4a N2. Endoscopic tunnel biopsies were obtained during the procedure.
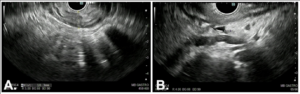
Figure 3. EUS: A – Gastric wall hipoechoic thickening (10 mm). B – Perigastric ascites and suspicious lymphnodes.
What is the most likely diagnosis?
Histopathological examination, including extensive immunohistochemical analysis (CK CAM5.2 positive, GATA3 positive, E-cadherin weak and incomplete, estrogen receptor positive, progesterone receptor negative, HER2 negative, Ki-67 20%), was consistent with gastric metastasis from breast lobular carcinoma.The case was discussed at a multidisciplinary oncology meeting, and neoadjuvant chemotherapy was initiated.
Discussion
Gastric metastases are uncommon, accounting for less than 2% of all gastric malignancies.1,2 Breast cancer is among the most frequent primary tumors metastasizing to the stomach, particularly invasive lobular carcinoma, which shows a distinctive propensity for gastrointestinal involvement.3,4 Other malignancies reported to metastasize to the stomach include melanoma, lung cancer, renal cell carcinoma, ovarian cancer, and esophageal carcinoma.1,2
Unlike ductal carcinoma, lobular breast cancer demonstrates a characteristic metastatic pattern with predilection for the gastrointestinal tract, peritoneum, and gynecologic organs.4 Gastric involvement often presents as diffuse infiltration of the gastric wall, mimicking primary linitis plastica, which may delay diagnosis due to nonspecific symptoms and inconclusive superficial biopsies.3
Endoscopic findings are frequently subtle or non-ulcerative, and imaging may show only diffuse wall thickening without focal lesions or significant metabolic activity on PET-CT.1,2 In this context, endoscopic ultrasound plays a crucial role in detecting transmural involvement, assessing locoregional lymph nodes, and guiding deep or tunnel biopsies Histopathological evaluation combined with immunohistochemistry is essential to differentiate primary gastric cancer from metastatic disease, as management and prognosis differ substantially.3
Treatment is generally systemic and guided by the primary tumor biology, emphasizing the importance of accurate diagnosis, with a key role for EUS, through a multidisciplinary approach.
References
- Oda I, Kondo H, Yamao T, et al. Metastatic tumors to the stomach: analysis of 54 patients diagnosed at endoscopy and 347 autopsy cases. Endoscopy. 2001;33(6):507–510.
- Menuck LS, Amberg JR. Metastatic disease involving the stomach. Am J Dig Dis. 1975;20(10):903–913.
- Taal BG, den Hartog Jager FC, Steinmetz R, Peterse H. The spectrum of gastrointestinal metastases of breast carcinoma: stomach involvement. Gastrointest Endosc. 1992;38(2):130–135.
- Borst MJ, Ingold JA. Metastatic patterns of invasive lobular versus invasive ductal carcinoma of the breast.Surgery. 1993;114(4):637–641.
Authors
Susana Marques1, Miguel Bispo1, Mariana Machado1, Ricardo Rio-Tinto1, Paulo Fidalgo1, Jacques Devière1,2
- Digestive Unit, Champalimaud Foundation, Lisbon, Portugal.
- Department of Gastroenterology, Hepatopancreatology, and Digestive Oncology, Erasme University Hospital – Université Libre de Bruxelles, Brussels, Belgium.


