US Quiz of the Month – Novembro 2022
CASE REPORT
We present the case of a 43-year-old female patient with past medical history of grade III obesity, thyroid nodules and an ovarian cyst, and history of a paternal aunt with pancreatic cancer at age 70. She was followed in Gastroenterology department due to an asymptomatic pancreatic lesion first described in a routine abdominal ultrasound when she was 35. The lesion was described as a nodular formation in the body-tail transition, mainly solid, with 67x49mm, with some liquid areas. Moving abroad in 2014, the patient lost surveillance and only returned to our department in 2019. At this time, laboratorial tests, CT scan and MRI were performed. Laboratorial tests revealed normal liver function as well as normal tumor markers (Ca 19.9, CEA, Neuron-specific enolase (NSE) and chromogranin A). CT scan showed a solid expansive formation relatively well delimited with tissue density and slight uptake with 42×32 mm of longest axis, presenting a cleavage plane with the liver and stomach. MRI (Fig. 1), revealed an 40x36mm heterogeneous, well delimited, nodule in the pancreatic body, hyperintense in T2 and hypointense in T1, compatible with a complex cystic formation, without communication with Wirsung (without dilatation). It had slight parietal enhancement and a nodular area with 15mm.
Endoscopic ultrasound (EUS) (Fig. 2) confirmed a nodular heterogeneous lesion, mainly solid, but with anechogenic areas, with well-defined contour, located in the pancreatic body, without upstream biliary or pancreatic duct dilation. An EUS-guided fine-needle aspiration (FNA) of the lesion was performed using a 22G needle (two passes).
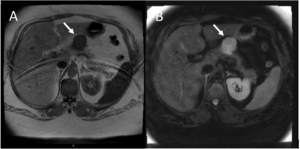
Fig. 1 – MRI with an 33x40x36mm heterogeneous, well delimited, nodule in the pancreatic body, hyperintense in T2 (B) and hypointense in T1 (A), compatible with a complex cystic formation, without communication with Wirsung (without dilatation)
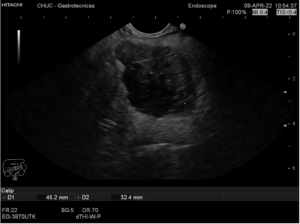
Fig 2 – EUS show a nodular heterogeneous lesion, mainly solid, but with anechogenic areas, with well-defined contour, located in the pancreatic body, without upstream biliary or pancreatic duct dilation
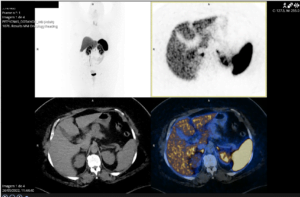
Fig 3 – PET 68Ga-DOTANOC without increase in somatostatin receptors
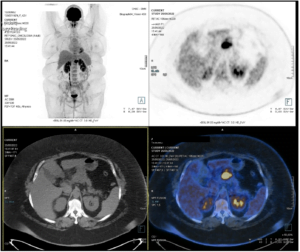
Fig 4 – PET-18 F-FDG was compatible with a malignant high metabolic grade lesion excluding distant capture
WHAT IS THE MOST LIKELY DIAGNOSIS?
DISCUSSION
Pathology showed cytomorphological alterations compatible with neuroendocrine tumor (NET), with positive immunostaining for CD56 and Ki67 <10%. The patient underwent a PET 68Ga-DOTANOC (Fig. 3) which showed no increase in somatostatin receptors and a PET-18 F-FDG (Fig. 4) which was compatible with a malignant high metabolic grade lesion, excluding distant capture. Given that, a laparoscopic body-tail pancreatectomy was decided and performed. The tumor morphology and the immunohistochemical study revealed a neuroendocrine tumor, well differentiated, Ki67 5%, corresponding to a low grade pancreatic NET (pNET), G2, pTMN T2N0R0. The patient is now proposed for surveillance with TC and PET-FDG.
Pancreatic incidentalomas represent a clinical entity increasingly recognized due to advances in and easier access to imaging techniques (1). Increasing numbers of pNET are being diagnosed incidentally in this context, most of which are non-functioning and malignant. They are relatively uncommon and although the most frequent age of diagnosis is in the fifth to seventh decade, it can appear at younger ages (2). EUS with FNA is an effective method to provide an accurate cytopathologic diagnosis in most patients (3). The combination of conventional radiological resources (MRI and CT) and nuclear medicine imaging (PET-CT Gallium-68 and PET-CT FDG) is mandatory for primary tumor diagnose, staging and defining the therapeutic strategy. Many published researches reported
that tumors with increased FDG accumulation seem more aggressive and correspond to bad prognosis (4). The appropriate treatment should be chosen based on the characteristics of the tumor, staging and associated comorbidities. The size, grade and tumor functionality are important to define who will be eligible for surgical treatment. In general, tumors bigger than 2 cm are eligible for surgical treatment, except insulinomas whose surgical resection is recommended no matter the size (5).
REFERENCES
1. Caban M., Malecka-Wojciesko E., et al. Pancreatic Incidentaloma. J Clin Med 2022 Aug 9; 11(16): 4648.
2. Gallotti A., Perez Johnston R., et al. Incidental neuroendocrine tumors of the pancreas: MDCT findings and features of malignancy. AJR Am J Roentgenol. 2013 Feb;200(2):355-62
3. Misra S, Saran RK, Srivastava S, Barman S, Dahale A. Utility of cytomorphology in distinguishing solid pseudopapillary neoplasm of pancreas from pancreatic neuroendocrine tumor with emphasis on nuclear folds and nuclear grooves. Diagn Cytopathol. 2019 Jun;47(6):531-540.
4. Gamal, G.H. The utility of 18F-FDG PET/CT in the diagnosis, staging of non-functioning pancreatic neuroendocrine tumors. Egypt J Radiol Nucl Med. 2021; 52, 234.
5. Falconi M, Eriksson B, Kaltsas G et al. Consensus guidelines update for the management of functional p-NETs (F-p-NETs) and non-functional p-NETs (NF-p-NETs). Neuroendocrinology. 2016; 103(2): 153–171.
AUTHORS
André Trigo1, Margarida Ferreira1, Clotilde Lérias1, Pedro Figueiredo1
1Gastroenterology Department, Centro Hospitalar Universitário da Universidade de Coimbra, Coimbra, Portugal.


